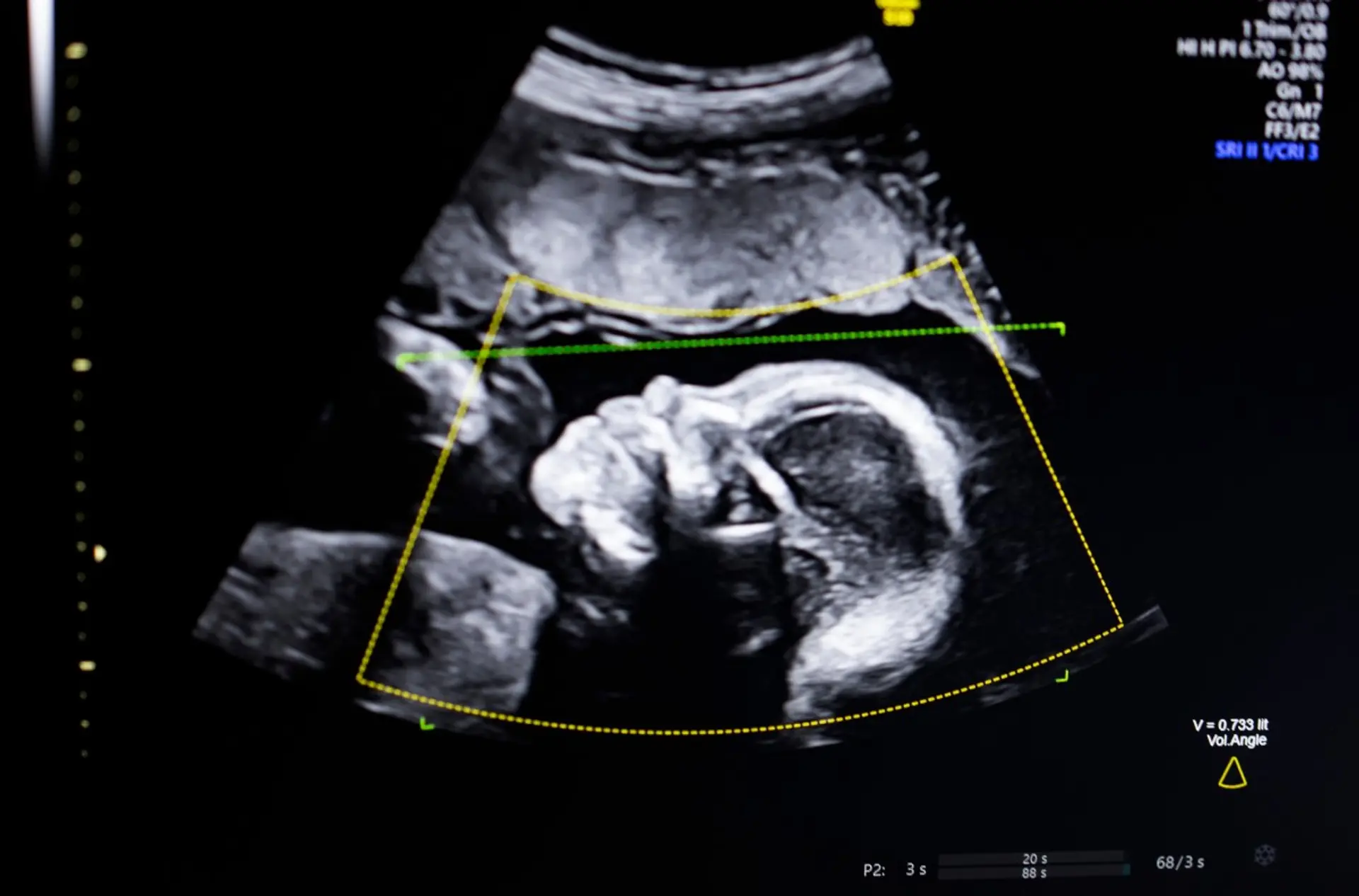
What happened
BioticsAI secured FDA clearance for its AI software, which assists in detecting fetal abnormalities within ultrasound images. This regulatory approval now permits the software, previously a TechCrunch Disrupt Battlefield 2023 winner, for clinical use. The clearance introduces a new, validated AI capability into diagnostic workflows, altering the operational conditions for medical imaging analysis by providing an approved automated assistance tool.
Why it matters
This FDA clearance introduces a new dependency on a regulated AI diagnostic aid for clinical operators and medical imaging departments. It increases the oversight burden on procurement and compliance functions to ensure proper integration, validation, and ongoing monitoring of this new AI software within existing clinical pathways. Furthermore, it tightens the dependency on external software providers for critical diagnostic support, potentially affecting system resilience and update cycles.
Related Articles

Confer Privacy-Focused AI Chat
Read more about Confer Privacy-Focused AI Chat →
Synthesia AI Voice Funding
Read more about Synthesia AI Voice Funding →
Runpod AI Cloud Revenue
Read more about Runpod AI Cloud Revenue →
Chai Discovery Eli Lilly Partnership
Read more about Chai Discovery Eli Lilly Partnership →